Technology
Cumulus has de-risked and shortened its battery development, using technology based on existing proven chemistries and architectures used daily in the metal-refining industry. These industrial Copper and Zinc electrowinning plants operate at ~100MWh equivalent scale, over lifetimes of 10-30 years. Consequently our battery configuration is already at grid-scale.
History
The first battery in modern times was invented by Alessandro Volta in 1799, using discs of Copper and Zinc as the electrodes, porous discs to keep the electrodes apart and brine as the electrolyte. The ‘Voltaic Pile’ Copper-Zinc battery was later developed into the Daniell cell, which was used extensively by the telegraph industry. These cells were primary (single-discharge) cells, which could not be recharged electrically: the cells had to be recharged physically with fresh chemicals.
In 2014, Cumulus developed a patented system for making Copper-Zinc rechargeable, using an ionically permeable separator, which was the significant missing component from Volta’s original battery.
The link with electrowinning is that every time the battery is charged, the Copper electrode releases Copper ions into the electrolyte and Zinc ions electro-win onto the Zinc electrode. On discharge the reverse happens. Providing the Zinc and Copper ions are prevented from migrating to the opposite electrode, the battery can be electrically charged and discharged for thousands of cycles.
Key Benefits
The Copper-Zinc battery offers several benefits, including:
- Scalability – its bipolar design means individual cells can be added in series to meet the desired voltage requirement
- Low cost – the lowest Levelized Cost of Storage (LCOS), for which the lowest chemistry capex cost is a key constituent.
- Low stress, low energy density, low voltage couple – low energy density is a desirable property for grid-scale energy storage because the cells will not be put under stress and will be inherently reliable
- Long life – lifecycle of 30 years with planned maintenance.
- Reliable – Cu/Zn chemistry is well understood and electrowinning plants have operated at ~100MWh scale for years
- Available – the very reliable battery chemistry and ease of maintenance provides 98% availability
- Efficient – with an anticipated round trip efficiency of greater than 80%
- Abundant materials – Copper and Zinc are the 25th and 26th most abundant materials in the earth’s crust. There are no constraints on Cumulus materials supply.
- Sustainable – the battery is 99.5% recyclable at the end of life.
The Cumulus Energy Storage Copper-Zinc battery is ideally suited for stationary bulk energy storage applications with a 4-12 hour charge and 4-12 hour discharge cycle where energy density is not an issue. Applications include commercial renewable electricity generation time-shifting, infrastructure security of supply (including off-grid) and electricity intensive industry energy management. Specific market opportunities include diesel engine generator set replacement, micro-grids, electric vehicle recharge infrastructure and mining.
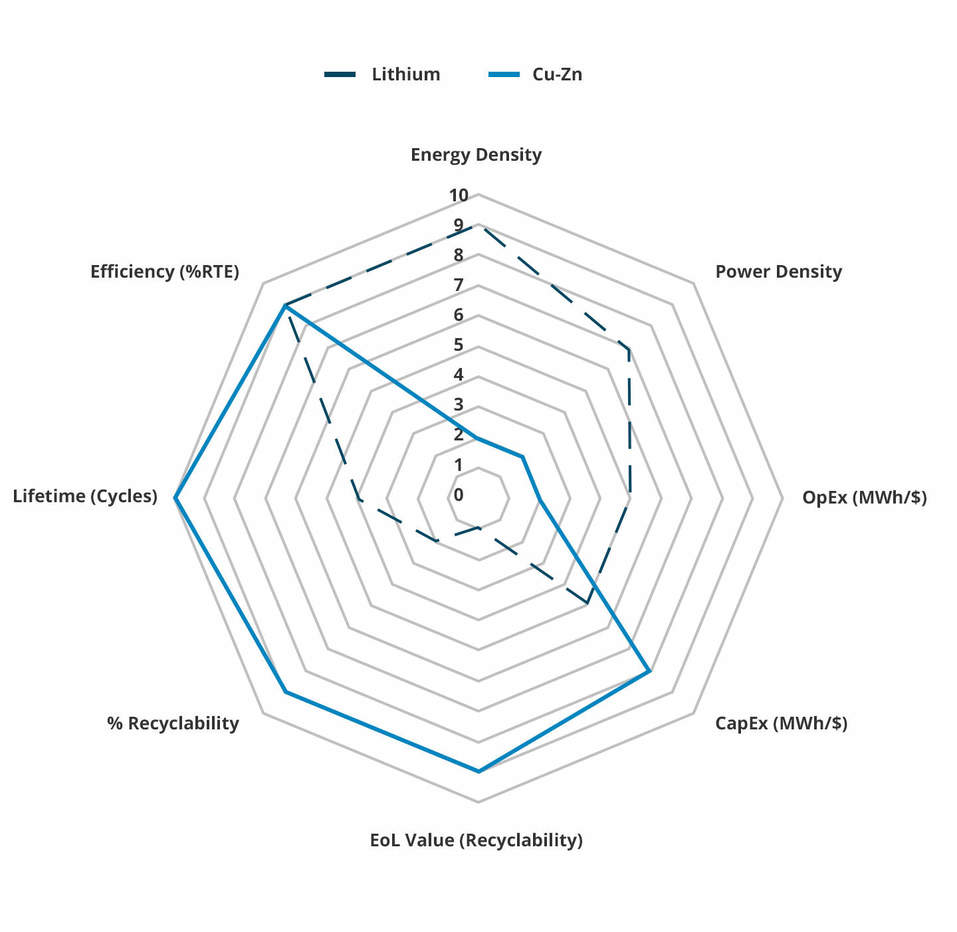
The following spider diagram compares and contrasts the properties of Lithium-ion and Copper-Zinc, to demonstrate how Copper-Zinc complements rather than competes with Lithium-ion.
Intellectual Property Rights
The rechargeable Copper-Zinc battery is patent granted in 15 countries with other international patents pending, which mean Cumulus Energy Storage is the only company in the world that can make and sell a bipolar rechargeable Copper-Zinc battery.